 One way to more rapidly develop an optimal flash purification method is to first run the target compound using thin layer chromatography (TLC) with different mobile phase compositions.
One way to more rapidly develop an optimal flash purification method is to first run the target compound using thin layer chromatography (TLC) with different mobile phase compositions.
The discovered optimal set of TLC conditions (stationary phase, mobile phase) can then be transformed directly into an optimal flash purification method. This technique is based on the relationship between the Rf (retention factor) of the compound as obtained in TLC, and its k (capacity factor) when applying the same separation conditions to the flash method.

The TLC essentially reveals how many column volumes (CV) it will take for the compound to elute via the flash method, using the relationship below:
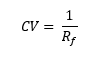
The accuracy of the method transfer depends on the quality of the TLC run. To prevent discrepancies it is vital to ensure your TLC is run under proper conditions with good procedure. A recently published paper [1] authored by my colleague Jack Silver discusses errors in the way TLC is sometimes taught. Mistakes in TLC procedure can impact Rf and therefore lead to an inaccurate calculation of the flash method. Jack's paper is an excellent primer not only for analysts new to TLC but a great refresher for experienced users as well. Using TLC to develop better flash methods is also a great way to save time, sample, and solvent.
For more information on TLC or our Chromatography products, visit https://www.teledyneisco.com/chromatography or contact us
References:
- Let Us Teach Proper Thin Layer Chromatography Technique!
Jack Silver
Journal of Chemical Education 2020 97 (12), 4217-4219
DOI: 10.1021/acs.jchemed.0c00437
https://pubs.acs.org/doi/10.1021/acs.jchemed.0c00437
